Publication Q&A: Factors Influencing Maternal Microchimerism Throughout Infancy and its Impact on Infant T cell Immunity
July 2022 – Blair Armistead, PhD, and Whitney Harrington, MD, PhD share their work in identifying previously unknown factors that contribute to the transfer of maternal cells that strengthen infant T cell responses to early childhood vaccines.
Christina Balle, Blair Armistead, Agano Kiravu, Xiaochang Song, Anna-Ursula Happel, Angela A. Hoffmann, Sami B. Kanaan, J. Lee Nelson, Clive M. Gray, Heather B. Jaspan and Whitney E. Harrington
Published July 1, 2022
Read this article in The Journal of Clinical Investigation.
What are the significant findings in this paper?
During pregnancy, maternal cells are transferred to the fetus and persist in small amounts during infancy and into childhood, a phenomenon known as maternal microchimerism (MMc). The factors that determine the number of cells a fetus acquires from the mother and how these maternal cells impact the fetal and infant immune system are largely unknown.
In our study, we show that the number of maternal cells transferred to the infant increased when the mother was not living with HIV, the mother and infant were more immunologically related, the infant was female and the infant was exclusively breastfed/chestfed. Further, we show that 7- and 15-week-old infants with higher levels of maternal cells at birth had more robust T cell responses to BCG, a vaccine given on the first day of life in the study population.
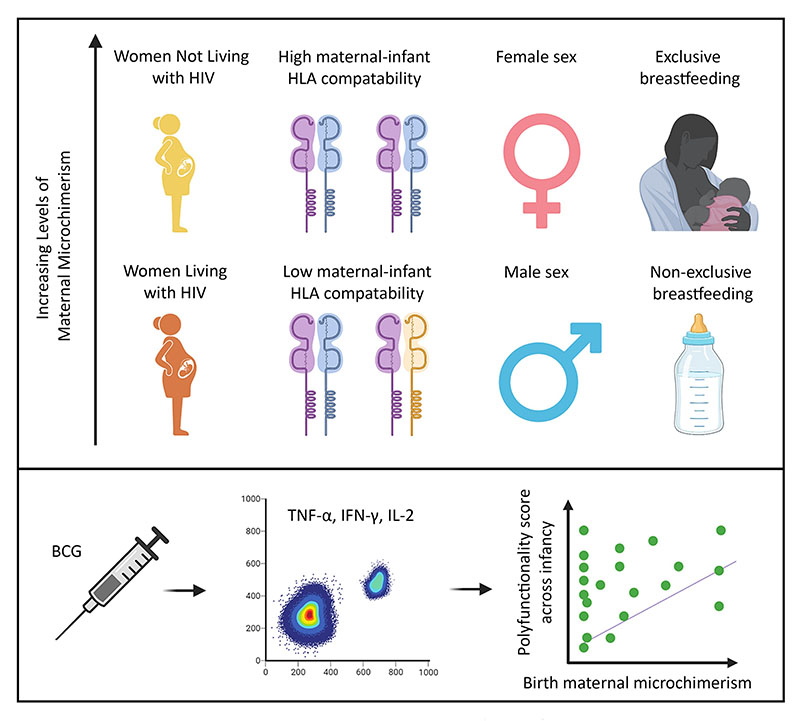
What does this research tell us that we didn’t know before?
We identify previously unknown factors that influence the number of maternal cells transferred to offspring during pregnancy and/or infancy and show that these maternal cells can strengthen infants’ T cell responses to a vaccine administered in early life.
What are the broad implications of this research?
Our research sheds light on maternal microchimerism as a key aspect of fetal and infant immune development and helps us understand the role of these maternal cells in preventing infectious diseases in early childhood.
What are the next steps and long-term goals for this research?
Future work is focused on identifying specific mechanisms that maternal cells use to influence or coordinate the fetal or infant immune responses to vaccination and common childhood infections. We also wish to explore breastmilk/chestmilk as a source of maternal microchimerism and study how maternal infection or vaccination may influence the type, specificity and function of maternal cells ingested by a nursing infant.
Any other specific information we should know about this paper?
We are grateful to our collaborators and all study participants who made this research possible.
Seattle Children’s contributing authors:
- Blair Armistead, PhD
- Whitney Harrington, MD, PhD
- Heather Jaspan, MD, PhD
- Chris Song
Learn more about Dr. Harrington’s and Dr. Jaspan's research.
