Out of Heartache, Hope Surfaces for Colton’s Metabolic Disorder
11.20.20 | Seattle Children's Press Team

Before his first breath, Colton Iverson had already received the gift of a lifetime. Just days old, he became the youngest patient to go on a drug recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of a life-threatening genetic condition called very long-chain acyl-CoA dehydrogenase, or VLCAD, deficiency.
For his parents, the hope it inspired did not come without heartache.
“Colton wouldn’t be here today without our first born, his older sister Cody,” said his mom, Lisa Iverson.
A few days after coming home as excited new parents of a healthy baby girl with an Apgar score of 10, Cody went lifeless in Lisa’s arms one morning. She and her husband, Ty Iverson, rushed Cody to their local hospital in northeastern Washington.
“They did everything they could to save her and they couldn’t,” Lisa said. “For about week, we had no idea what happened to Cody.”
On the day of Cody’s service, the Iversons heard from their family medicine doctor, Dr. Geoffry Jones.
“As we were driving home, I remember the exact spot on the road when we got the call from Dr. Jones,” Lisa said. “He said her newborn blood test showed she had a genetic condition called VLCAD. He recommended we get in touch with a specialist at Seattle Children’s to learn more.”
VLCAD: A life-threatening metabolic block
 VLCAD deficiency is an inherited condition that prevents the body from converting certain fats to energy.
VLCAD deficiency is an inherited condition that prevents the body from converting certain fats to energy.
“The body first uses stored sugar for energy because it’s the easiest to break down. As your sugar reserves drop, your body shifts to using fat as a source of fuel,” said Dr. Lawrence Merritt, a biochemical geneticist who treats patients with metabolic disorders at Seattle Children’s. “Fats are units of carbon and they come in short, medium and long-chain fats depending on how many carbon units they have. To convert fat into energy, your body breaks down the chains two carbon units at a time.”
Children with VLCAD do not make the enzyme needed to break down long-chain fats. As a result, they are unable to use this type of fat for energy.
The rare deficiency affects about 1 in 70,000 to 100,000 children. Expanded newborn screening introduced in 2008 makes early detection of VLCAD in Washington state possible. Without prompt diagnosis and treatment, a child’s blood sugar will drop significantly. In severe cases, it is life-threatening, leading to liver failure, heart failure and coma.
From heartache to hope
 Lisa and Ty first traveled from their home near the Washington-Idaho border to meet Merritt in Seattle a few months after Cody’s passing. He explained how Cody had such a severe form of VLCAD that there was nothing more they could have done.
Lisa and Ty first traveled from their home near the Washington-Idaho border to meet Merritt in Seattle a few months after Cody’s passing. He explained how Cody had such a severe form of VLCAD that there was nothing more they could have done.
“I let them know they had a one in four chance of this happening again,” Merritt said. “I wanted them to get in touch if they decided to have kids in the future so we could look at all possibilities and options.”
About a year and a half later, the Iversons learned they were pregnant. Lisa underwent prenatal testing to look for genetic disorders. Again, she received a fateful call from Dr. Jones.
“He said there are two things we know for sure,” she said. “Number one, you’re having a boy and number two, he’ll have VLCAD.”
The news was devastating to the expecting parents.
“Ty and I both felt sorry for ourselves,” Lisa said. “I was crying when I felt Colton kick me. It was the first time I felt him and it was a pretty strong kick. It was our wake-up call. We knew then we needed a plan because he was here with us.”
When the couple reconnected with Merritt, he had an idea. A company was developing a new drug called triheptanoin for pediatric and adult patients with long-chain fat disorders. He thought it might offer hope for Colton if they could start treatment immediately after birth.
“Colton had the two mutations we knew would run in his family and we anticipated he was going to be severely affected,” Merritt said. “I wanted him transferred to Seattle Children’s neonatal intensive care unit (NICU) the day he was born. We could treat him from the beginning and keep him from going into crisis.”
A new alternative to treating VLCAD
 The standard treatment for VLCAD deficiency is to restrict fasting to just a few hours. This provides the body with enough sugar reserves so it doesn’t need to tap into the fat for energy. Avoiding fasting is often combined with a low-fat/high-carbohydrate diet.
The standard treatment for VLCAD deficiency is to restrict fasting to just a few hours. This provides the body with enough sugar reserves so it doesn’t need to tap into the fat for energy. Avoiding fasting is often combined with a low-fat/high-carbohydrate diet.
Children with VLCAD deficiency also take MCT oil, a supplement made from a type of fat called medium-chain triglycerides.
“Since they only block breaking down long-chain fats, we give them extra medium-chain fats as an alternative source of fuel” Merritt said.
Triheptanoin offers an alternative to MCT oil. Merritt thought it had benefits in a case as severe as Colton’s.
“The body metabolizes triheptanoin differently than MCT. It appeared to offer a better way to provide energy in a patient with VLCAD.”
Merritt lobbied the FDA and Ultragenyx Pharmaceutical Inc., the company developing the drug, to authorize an emergency use of the drug for Colton before he was ever born.
“It had never been done before, but I believed I had a strong case given the severity of the mutation and knowing his sister’s tragic outcome,” he said. “I was willing to jump through whatever hoops I had to so Colton could start this drug within his first few days of life.”
Colton’s arrival
While Merritt awaited final approval, Ty and Lisa relocated to Seattle to prepare for Colton’s arrival. Three days before their due date, they learned the FDA had granted them emergency use of triheptanoin.
“Everything changed for us that day,” Ty said. “We put all our trust into Dr. Merritt and he has always gone above and beyond for Colton. I don’t know what our lives would look like without him.”
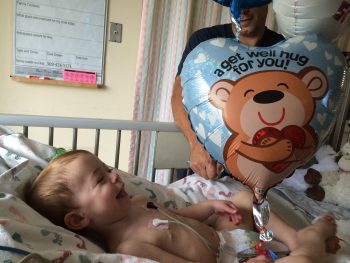
As planned, Colton was born at UW Medical Center and transferred to Seattle Children’s that same day. A photo of Cody watched over his crib in the NICU as the first drops of triheptanoin entered his bloodstream.
“Before Colton, only three other older children had ever tried triheptanoin,” Lisa said. “Not knowing how Colton would do, we definitely had our hesitations. We believe Cody got him through that. She was there protecting him the whole time.”
Six years later
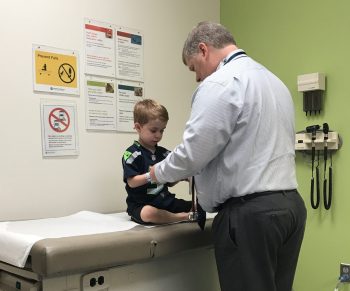
 One glance at Colton today and he’s your normal, healthy 6-year-old. He loves fostering rescue kittens, sharing secrets with his grandma, and NFL football. From the outside, you’d never know he lives with a serious medical condition.
One glance at Colton today and he’s your normal, healthy 6-year-old. He loves fostering rescue kittens, sharing secrets with his grandma, and NFL football. From the outside, you’d never know he lives with a serious medical condition.
“We’re so happy to report Colton has only thrived since birth,” Lisa wrote in recent Facebook message.
Since his first week in the NICU, Colton has progressed from receiving triheptanoin on a constant drip through a feeding tube to every 20 minutes to every hour to every few hours. The Iversons worked closely with Melissa Gunnarson, a dietitian at Seattle Children’s, to find new ways to incorporate the drug with his meals and to adjust his calories needs as he grew.
Now, he takes a tablespoon of the oily liquid three times a day. He avoids fasting and stays on a low-fat diet.
Colton sees Merritt for checkups twice a year. Most recently, due to COVID-19, they did a telehealth visit from their home in Bonner’s Ferry, Idaho.
A long-term worry for VLCAD is that long-chain fats build up in tissues causing heart and liver damage. Merritt says data from clinical trials suggest triheptanoin may benefit the heart, liver and muscles too. From Colton’s experience and that of other patients he’s treated with triheptanoin, Merritt sees the potential to transition more patients to the drug if their symptoms progress.
“Colton has never had low blood sugar since he was born, he’s never had liver dysfunction and his heart is completely normal,” he said. “Other patients who are doing well on standard treatment until their hearts start to get worse, we have then we put them on triheptanoin and their hearts have improved.”
The miracle of 2020
 In a year that carries great loss and upheaval, the Iversons found reason to celebrate when the FDA approved triheptanoin for children with Colton’s condition in June 2020.
In a year that carries great loss and upheaval, the Iversons found reason to celebrate when the FDA approved triheptanoin for children with Colton’s condition in June 2020.
Although Colton transitioned to receive the drug as part of an ongoing research study once emergency use expired, his parents feared what might happen if the trials weren’t successful.
“He’ll need this drug the rest of his life so we’re incredibly grateful that we don’t have to worry about Colton losing access to this drug,” Ty said. “From the entire metabolic team at Seattle Children’s to the drug company that went after a drug for a rare condition like VLCAD, we’re so thankful.”
Merritt also marked the drug’s approval as a victory for his young patients.
“This drug has potential for broad use in these disorders,” he said. “I kept reading over the label for restrictions limiting its use in patients with disorders like Colton’s. They didn’t put any limits on it by age and I like to think that’s because of Colton and his remarkable story.”